Информация за изтегляне на медицинско изделие
Устройства за грижи за съня и дихателната система на Philips Respironics
Информация за клиницисти, всичко на едно място
През юни 2021 г. след откриване на потенциален риск за здравето, свързан с част в определени CPAP, BiPAP и механични вентилаторни устройства, Philips публикува доброволно съобщение относно безопасността на място (извън САЩ)/известие за доброволно изтегляне (само за САЩ).
Ангажирани сме да поддържаме клиницистите по време на пълния процес на отстраняване на несъответствие и ще Ви предоставим набор от ресурси, които да спомогнат за по-добро информиране, инструктиране и подкрепа на Вашите пациенти. Посещавайте редовно страницата за най-актуална информация за Вас и Вашите пациенти.
Благодарим Ви за търпението и непрекъснатото доверие.
Ресурси
Помолихме Вашите пациенти да предприемат следните стъпки:




Обадете се на 877-907-7508
Новини и актуална информация
Цялата актуална информация за клиницисти
Въпроси и отговори
Следните посочени продукти са засегнати от известието за изтегляне/съобщението относно безопасността на място:
CPAP и BiLevel PAP устройства
Всички засегнати устройства, произведени преди 26 април 2021 г., всички серийни номера на устройства
Непрекъснат вентилатор, минимална вентилаторна поддръжка, използване от здравно заведение
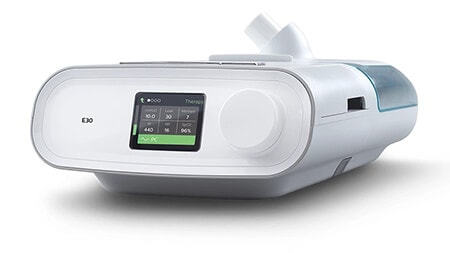
E30
(Разрешение за употреба при спешен случай)

DreamStation ASV
Също познат катоDreamStation BiPAP autoSV

DreamStation ST, AVAPS
Също познати като DreamStation BiPAP AVAPS,DreamStation BiPAP S/T

SystemOne ASV4
Също познати като System One BiPAP autoSV,System One BiPAP autoSV Advanced

C серия S/T, AVAPS
Също познати като System One BiPAP AVAPS (C-Series),System One BiPAP S/T (C-Series)

OmniLab Advanced Plus
Устройство за титриране в лабораторни условия
Непрекъснат вентилатор, минимална вентилаторна поддръжка, използване от здравно заведение

A-серия BiPAP Hybrid A30
Познат също катовентилаторBiPAP Hybrid A30 (A-серия)
(не се продава в САЩ)

Вентилатор A-серия BiPAP V30 Auto
Познат също като вентилатор BiPAP V30 Auto(А-серия)
Непрекъснат вентилатор, неживотоподдържащ

Серия A BiPAP A40
Познат също като вентилатор BiPAP A40 (А-серия)
(не се продава в САЩ)

Серия A BiPAP A30
Познат също катовентилаторBiPAP A30 (A-серия)
(не се продава в САЩ)
Ако устройството Ви е засегнато...
Кои продукти не са засегнати и защо?
Продуктите, които не са засегнати, може да имат различни материали за звукопоглъщаща пяна, тъй като с течение на времето стават достъпни нови материали и технологии. Също така звукопоглъщащата пяна в незасегнатите устройства може да бъде поставена на различно място поради дизайна на устройството.